 Last year I was fortunate enough to be one of the winners of an Accu-chek mobile during a competition held by Roche Diagnostics. I have now been using the meter for about 5 months so thought it was time to share some of my impressions.
Last year I was fortunate enough to be one of the winners of an Accu-chek mobile during a competition held by Roche Diagnostics. I have now been using the meter for about 5 months so thought it was time to share some of my impressions.
The meter is available in all the usual places and at the time of publication the RRP was $150. Strips are a different story. In Australia, strips are only available through the NDSS to people who use insulin. This is the only time I’ve seen a restriction of this kind, and makes me think that the full unsubsidized costs of consumables for this meter is considerably higher than for any of the others in the market.
Advantages:
Well, there is only one main but HUGE advantage on this meter…. it’s all in one!.
This meter doesn’t really use strips like most meter. It uses a cassette that contains 50 tests. Think of it as a continuos strip 50 times as long, that rolls around a casette like an audio tape (sorry, couldn’t find an analogy from the ipod era). You install the casette, and the meter tells you how many tests remain, when it’s empty you just open the meter, take the old one out and put a new casette in. very cool….
Accu-check has also integrated a modified version of their multi-clix lancet device which provides 6 lances into once cartridge. It’s attached to the meter (although it can be detached if needed) and can be operated with the same hand that holds the whole unit. It’s perfect for one handed testing… Especially when driving… not that I would ever do that…
All this integration makes the traditional meter case completely obsolete. I now carry my meter in my pocket much like I would carry a mobile phone. In fact Accu-chek gave me a leather cover that reminded me of the mobile phone covers from the 90’s.
In addition to this, there are a few extra features… display is bright yellow over black, so no back-light is necessary even in the darkest places (assuming you can find the tip of the test area to put blood in), has all the averages and other stats I have never used, and supports alternative site testing (AST) so you can give your fingers a break ( Thanks to whoever invented this, I can’t see myself ever testing in my fingers again )
Disadvantages:
There are a couple if minor disadvantages that I’m happy to live with. The first one is that you don’t carry a meter case anymore, so you’ll have to find a different place for things like hypo fixes and spare supplies. The second disadvantage is that it makes a bit more noise than traditional meters, mainly because of all the moving parts. The last one is its size… this is not a small meter by any means, but in my case I find it compensates with the fact that you don’t carry a case anymore.
Verdict:
This is probably the best meter I’ve tried so far and thus why I carry it every day. It’s not perfect but definitely makes testing 8 or 10 times a day a lit easier that most of the other meters I’ve tried.




 Posted by Henry
Posted by Henry 









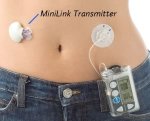 According to the study “The Medtronic MiniMed Subcutaneous Glucose Sensor was originally approved by the FDA for commercialization as part of the Continuous Glucose Monitoring System (CGMS) on June 15, 1999 (PMA 980022). The Sensor is composed of a microelectrode with a thin coating of glucose oxidase beneath several layers of biocompatible membrane. This same sensor is used as part of the Guardian REAL-Time System, the latest advance in continuous glucose monitoring, which is based on the CGMS. Similar to the CGMS, the Guardian REAL-Time System has been developed for use in conjunction with a standard home blood glucose meter. The Guardian REAL-Time received regulatory approval from the FDA in 2006. As currently used, the Subcutaneous Glucose Sensor is labeled for a maximum use duration of 72 hours, using only the abdomen area as an insertion site. Recent studies have shown that the useful sensor life could extend beyond three days, and it is reasonable to expect a significant percentage of sensors to last six days. It is the goal of this study to confirm sensor performance accuracy data from one of these recent studies. The sensor is also commonly worn in body areas other than the abdomen (such as the buttock). This study will also demonstrate sensor accuracy when used in an alternate site.”
According to the study “The Medtronic MiniMed Subcutaneous Glucose Sensor was originally approved by the FDA for commercialization as part of the Continuous Glucose Monitoring System (CGMS) on June 15, 1999 (PMA 980022). The Sensor is composed of a microelectrode with a thin coating of glucose oxidase beneath several layers of biocompatible membrane. This same sensor is used as part of the Guardian REAL-Time System, the latest advance in continuous glucose monitoring, which is based on the CGMS. Similar to the CGMS, the Guardian REAL-Time System has been developed for use in conjunction with a standard home blood glucose meter. The Guardian REAL-Time received regulatory approval from the FDA in 2006. As currently used, the Subcutaneous Glucose Sensor is labeled for a maximum use duration of 72 hours, using only the abdomen area as an insertion site. Recent studies have shown that the useful sensor life could extend beyond three days, and it is reasonable to expect a significant percentage of sensors to last six days. It is the goal of this study to confirm sensor performance accuracy data from one of these recent studies. The sensor is also commonly worn in body areas other than the abdomen (such as the buttock). This study will also demonstrate sensor accuracy when used in an alternate site.” This week I had the opportunity to attend the Insulin Pump Expo 2009. This event is organized by The local chapter of Diabetes Australia, and brings together the diabetes community interested in Insulin Pumps. There were two presentaton tracks on the day, one for prospective pumpers and an advanced one for those of us who have been pumping for a while.
This week I had the opportunity to attend the Insulin Pump Expo 2009. This event is organized by The local chapter of Diabetes Australia, and brings together the diabetes community interested in Insulin Pumps. There were two presentaton tracks on the day, one for prospective pumpers and an advanced one for those of us who have been pumping for a while. Many people with chronic conditions successfully manage them with pills. This, however, is not the case for those of us living with Type 1 diabetes.
Many people with chronic conditions successfully manage them with pills. This, however, is not the case for those of us living with Type 1 diabetes.






